Case Study – A Class 2 Medical Device Product Development
Background
In early 2019 we were approached by Memphasys and asked to submit a quotation for supply of their Felix Cartridge assembly.
The following is a description of the Felix device from www.memphasys.com

The Felix™ device for sperm separation
The Felix™ device is an automated electrophoretic system. The Felix™ device separates sperm from raw semen using a proprietary process which combines electrophoresis and size exclusion membranes. Sperm are harvested with exclusion of cellular contaminants such as leukocytes and precursor germ cells.
The Felix™ device consists of two main components: a console, which supplies electrical power, and a sterile disposable cartridge for sperm isolation and selection.
The Felix™ device is the first commercial product to be developed by Memphasys. The Felix™ device is currently being sold in international markets whilst clinical study and/or regulatory processes are underway.
After submitting the quotation and several follow up meetings, we were awarded the project and implemented our usual project management process.
All details of the many components in the assembly were populated into our project plan template and a project team was established.
The first of our suppliers to be engaged in the project was our tool making partner in Malaysia.
Whilst they worked with our tooling engineers to create a design for manufacture document (DFM) for each of the plastic moulded parts our procurement team was in discussion with the many suppliers of purchased parts and materials.
Design for Manufacture (DFM)
There are five injection molded parts in the assembly, two of which are over molded with a soft plastic resin that creates a sealing feature in place of a gasket.
Each of the parts looked in the first instance, quite simple, but nevertheless, we followed our usual procedure and conducted a full DFM which included mold flow simulation.
Following procedure, the engineer that manages our mold flow studies, worked with some our senior engineers to judge the best position for the gates (the point at which the plastic enters the mold cavity) and set the study parameters.
The results that were returned was unexpected and justified the time spent doing the studies.
Over Mold Part 1
The mould flow simulation predicted a cavity fill time that was more than was expected and required a higher inject pressure than anticipated so the team agreed that the position of the gate should be moved and the engineer ran the study again.
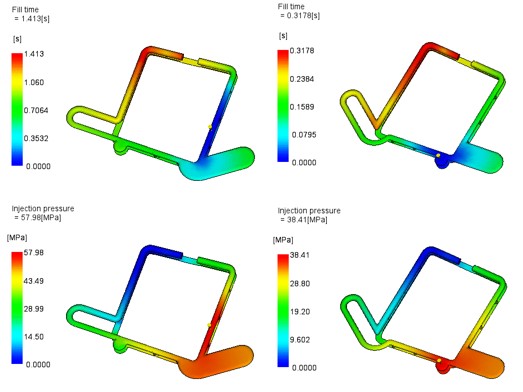
Results of Mould Flow Simulation Study Not representative of the final part/gate design
This time, the results were as originally expected. The injection pressure was low and consequently the cavity fill time was within the ideal range.
The application of the results of this computer simulation resulted in a trouble-free validation of the mold when it was trialed.
Over Mold Part 2
The mold flow simulation predicted that the flow of plastic while filling the cavity would result in a weld line (joining of the ends of the flow front) in a position that undoubtedly cause trapping of the air and gasses that should otherwise be expelled through vents. The position of this weld was such that the gasses could not be vented and would cause a defect in the part.
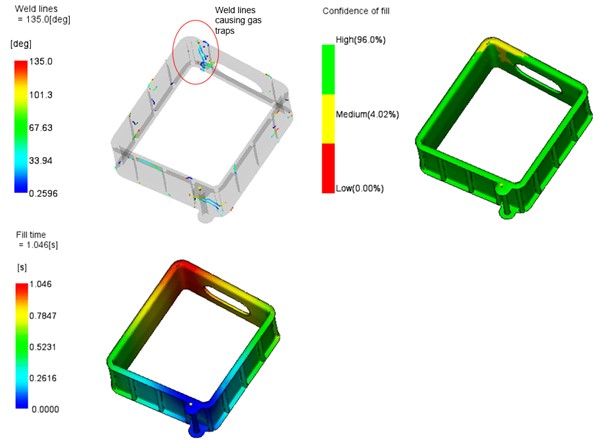
Results of Mould Flow Simulation Study Prediction of gas traps rectified by moving gate position.
Once again, iterative studies were done with different gate positions, and this resulted in a perfect parts from the first tool trial.
The customer was complimentary as they understood that we had solved potential moulding issues before tool design had even started.
Of note is that all simulations and studies are performed in house on dedicated servers to deliver short lead times and cost benefits to our customers.
Materials and Part Sourcing
Memphasys and their contract design engineers, Hydrix, had done a lot of research to find appropriate suppliers of the materials and purchased parts which made our job of purchasing these quite easy. However, as the project progressed new requirements cleanliness were added and our team worked through these with the suppliers. In some cases, materials originally specified no longer met the requirements and new materials and suppliers had to be sourced.
A plated pressed metal part required significant redesign and our engineers worked closely with the supplier and the original design engineer to determine a specification that worked in the assembly and was manufacturable. This process was controlled and tracked by a DFM.
Our ISO13485:2016 Quality Management System for medical device manufacture ensures effective document control internally and for purchased materials and parts.
Scientific Moulding and Validation
The tools were fabricated by our supplier in Johor, Malaysia under the watchful eyes of our project team including our engineers from our Malaysia factory who were able to make weekly visits to inspect the progress and be available to answer any questions that the supplier may have.

When complete, the tools were trialed in one of our Engel injection moulding machines in our Malaysia factory and first article inspections (FAI) were completed to ensure that the part dimensions and aesthetics met the specification.
Some tuning of the tools was done and once satisfied that they were operating correctly and parts met the specification, they were signed off for shipment to Australia.
After arriving in Sydney, each of the tools underwent our Scientific Moulding Procedure (SMP) which involves a series of experiments to determine the most suitable process settings for the plastic resin and part design. The SMP process outputs a “process window” which, if the process settings stay within, will always produce good parts.
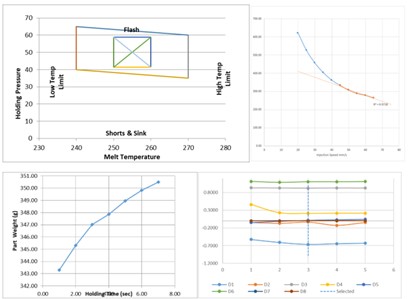
When the process is complete these settings are “locked in” and only a very few authorized people can approve any changes via a documented process.

Measuring a part – not related to this project
A sample batch of parts were molded, inspected, and measured in our QC laboratory
Measurements were evaluated by one of our NPI engineers and after calculating that the data statistically proved that the parts will consistently meet the specification the tools were approved for production.
Clean Cell Moulding and Clean Room
The Felix cartridge is a medical device used in IVF laboratories to assist with “start of life” and therefore particle and biological contamination must be controlled.
A clean moulding cell was also built to control particle contamination during the moulding cycle, and we built an ISO Class 7 Clean Room specifically to assemble the cartridge in.

Clean Molding Cell & Clean Room
Process Flow
The Felix Cartridge assembly contains quite a few parts including:
- 5 plastic injection molded parts
- 2 over molded plastic injection molded parts
- 2 pressed metal and plated parts
- 2 “fabric” membranes
- 1 chemical membrane
- Several labels
- A sterilization pouch
- Several carton boxes
Plastic parts are molded within the previous mentioned clean cell, placed in double layer poly bags, sealed which are stored and transported in plastic creates.
There are several process undertaken within the clean room.
A chemical membrane is manufactured by mixing a powder with a liquid, screeding a very accurate volume of the mixture onto a glass plate, cured in an oven and cut to the required size.
Fabric membranes are manufactured by cutting the bulk fabric to the correct size and fusing a bezel onto the edges.
Prior to entering the clean room, the plated pressed metal parts are thoroughly cleaned and prepared.
The plastic parts are taken to the clean room where they are assembled with the other parts and once assembled, packed in a temporary pouch and sealed.
The finished assembly is sent to a local supplier where it is Gamma irradiated to further ensure that there is no biological contamination and returned to the clean room where it is sealed in its final packaging ready for distribution to IVF laboratories all around the world.
Traceability
The Felix Cartridge is being registered with the FDA as a class 2 medical device and therefore requires complete traceability of all materials and components.
W&S systems ensure that this is possible. The batch number can be entered into our MRP system, PRONTO, and a report is created providing the details of all materials. We can drill down to the batch numbers of all components and materials with the cartridge assembly. The injection moulding machine, its process settings, the operator that packed the parts, the time that the parts were mouded are all details that the system captures.
Master Supply Agreement
Due to the complexity of the project, W&S and Memphasys agreed that a contract, or master supply agreement was required, and the content of this document were negotiated and signed in due course.
With plastic injection moulding tooling, process settings, equipment, and legal agreements all in place, W&S and Memphasys look forward to helping many women achieve their dream of having a child.
